Draw the lewis structure for the polyatomic trisulfide – Drawing the Lewis structure for the polyatomic trisulfide embarks on an illuminating journey into the realm of molecular architecture, unraveling the intricate dance of electrons within this fascinating chemical entity.
The subsequent discourse will delve into the intricacies of trisulfide’s Lewis structure, exploring the distribution of valence electrons, identifying key atoms, and elucidating its molecular geometry and properties. Through a comparative analysis with other polyatomic ions, we shall uncover the distinctive features that set trisulfide apart.
Definition of Trisulfide
Trisulfide is a polyatomic ion with the chemical formula S 32-. It consists of three sulfur atoms covalently bonded to each other. Trisulfide is a common ligand in coordination complexes, and it can also be found in some minerals.
- Examples of polyatomic trisulfides include:
- Sodium trisulfide (Na 2S 3)
- Potassium trisulfide (K 2S 3)
- Ammonium trisulfide ((NH 4) 2S 3)
Lewis Structure of Trisulfide
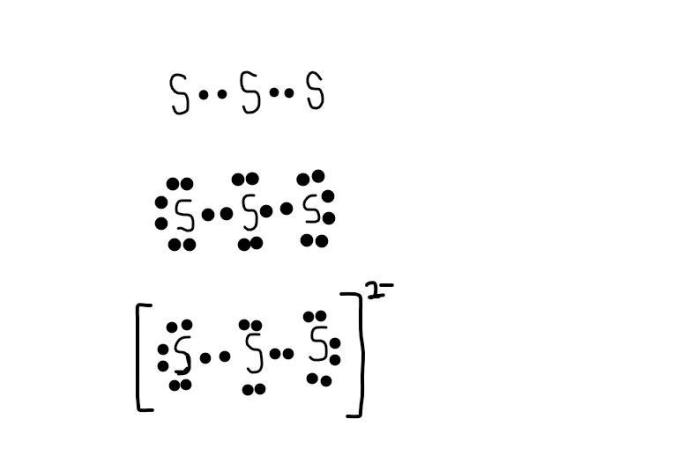
The Lewis structure of trisulfide shows the arrangement of valence electrons in the ion. To draw the Lewis structure, we first need to determine the total number of valence electrons in the ion. Sulfur has six valence electrons, and there are three sulfur atoms in the ion, so the total number of valence electrons is 18.We
then place the sulfur atoms in a triangular arrangement, with each sulfur atom bonded to the other two sulfur atoms. We then add the remaining valence electrons to the sulfur atoms, placing them in pairs. The final Lewis structure of trisulfide is:
:S: / \ :S---S: \ / :S:
The central atom in the trisulfide molecule is the sulfur atom that is bonded to the other two sulfur atoms. The terminal atoms are the two sulfur atoms that are bonded to the central sulfur atom.
Molecular Geometry of Trisulfide
The molecular geometry of trisulfide is trigonal planar. This geometry is predicted by VSEPR theory, which states that the shape of a molecule is determined by the repulsion between the valence electron pairs in the molecule. In the case of trisulfide, the three valence electron pairs around the central sulfur atom are arranged in a trigonal planar geometry to minimize the repulsion between them.
Other molecules with a trigonal planar molecular geometry include boron trifluoride (BF 3), carbon dioxide (CO 2), and nitrate ion (NO 3–).
Properties of Trisulfide
Trisulfide is a yellow-orange solid that is soluble in water. It is a strong reducing agent and can react with a variety of oxidizing agents. Trisulfide is also a good nucleophile and can react with electrophiles.
Trisulfide is used in a variety of applications, including:
- As a reagent in analytical chemistry
- As a reducing agent in the production of dyes and other chemicals
- As a sulfur source in the production of fertilizers
Comparison to Other Polyatomic Ions: Draw The Lewis Structure For The Polyatomic Trisulfide
Trisulfide is similar to other polyatomic ions, such as sulfate (SO 42-) and nitrate (NO 3–), in that it is a negatively charged ion that is composed of multiple atoms. However, trisulfide differs from these other ions in its molecular geometry and its properties.
The following table summarizes the key differences between trisulfide, sulfate, and nitrate:
| Property | Trisulfide | Sulfate | Nitrate |
|---|---|---|---|
| Molecular geometry | Trigonal planar | Tetrahedral | Trigonal planar |
| Color | Yellow-orange | Colorless | Colorless |
| Solubility in water | Soluble | Soluble | Soluble |
| Reducing ability | Strong | Weak | None |
FAQ Insights
What is the chemical formula of trisulfide?
S3
How many valence electrons does trisulfide have?
24
What is the hybridization of the central sulfur atom in trisulfide?
sp3
